Abstract
Introduction:
Reactivation of viruses such as cytomegalovirus (CMV) or Epstein Barr virus (EBV) after allogeneic hemopoietic stem cell transplant (aHSCT) is associated with increased non relapse mortality and a requirement for antivirals with mainly hemopoietic and renal toxicities that can further compromise transplant outcomes and increase health care costs. The use of 3 rd party virus specific T cells (VSTs) has been effective in treating recurrent and refractory viral reactivation after transplant and leads to rapid restoration of viral immunity. We investigated whether early administration of 3 rd party VSTs together with antiviral therapy could safely enhance immune recovery and improve viral control in patients requiring treatment for their initial CMV and EBV infection after aHSCT.
Methods:
We performed a single arm phase 1 clinical trial in which aHSCT patients requiring treatment for their first CMV or EBV reactivation (or EBV driven malignancy) received infusions of partially HLA matched 3 rd party VSTs within 7 days of commencing standard antiviral treatment. Patients were required to have a viral copy number of at least 1,000 copies/mL for CMV, 10,000 copies/mL of blood for EBV, or proven tissue infection irrespective of copy number for treatment initiation. T cell products were expanded from G-CSF stimulated aphereses from normal donors following peptide stimulation and CD137 magnetic bead selection. T cells were cultured for up to 12 days before specificity testing and cryopreservation. Patients were eligible to receive up to 4 doses of VSTs at 4 week intervals with products selected with a minimum of 1 of 6 HLA matches at HLA-A, -B and -DRB1 with antiviral activity demonstrated through the shared HLA molecule. The primary endpoint of the study was infusion safety.
Results:
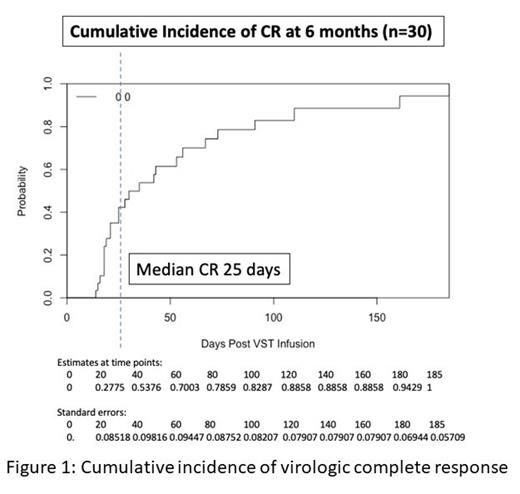
Thirty aHSCT patients were treated with 1-4 VST infusions (27 CMV, 3 EBV) commencing at a median of 4 days after initiation of antiviral treatment. 27 patients were transplanted for hematological malignancies, 3 for immune deficiencies. Conditioning was myeloablative in 12 patients and the majority of patients (22/30) received in vivo T cell depletion. 7/27 CMV seropositive recipients were transplanted from CMV seronegative donors. A total of 41 infusions were given, most frequently targeting antigens presented through shared HLA molecules A2 and/or B7. All infusions were administered at a cell dose of 2 x 10 7/m 2. VST products were CD3 + (median 97.9%, range 96.2 - 99.4%), with median percentage of CD3 +CD8 + 85.8% (range 23.2 - 95.5%). There was one infusion related adverse event consisting of fever that resolved rapidly after admission and antibiotics. Overall viral response rate was 100% with a complete response rate of 94% (Figure 1). Of the 28 patients who achieved a CR (after either 1 or 2 infusions), 22 remained virus PCR negative (n = 8) or below the limit of quantitation (n = 14) for the duration of follow up. 3 patients had brief episodes of quantifiable reactivation not requiring additional therapy and 3 patients required a second infusion following initial CR. All remained PCR negative after their 2 nd CR. All 3 patients treated for EBV PTLD achieved sustained CR. Overall rates of acute and chronic GVHD post-infusion were 33% (10/30) and 20% (6/30) respectively (grade IIII/IV aGVHD 10%, severe cGVHD 7%). VST infusion was associated with a reduction in activation and inhibitory marker expression on CD4 + and CD8 + lymphocytes within the first 30 days and recovery of CD8 + (and more slowly CD4 +) CD45RA -CD62L - effector memory cells. Within the first 100 days after infusion there was an increase in interferon-γ responsiveness of blood lymphocytes. CMV and EBV specific tetramer positive T cells were detected comprising up to 13% of CD8 + cells for up to 6 months post infusion. At 1 year post transplant, non relapse mortality was 10%, cumulative incidence of relapse was 7% and overall survival was 87%.
Conclusion:
The combination of traditional antiviral treatment and early administration of 3 rd party VSTs is safe and achieves high rates of viral control without evidence of increased acute or chronic GVHD and with evidence of enhanced immune responses to viral antigens. 1 year overall survival was high with low rates of non relapse mortality and relapse. These encouraging results require confirmation in a prospective randomized study comparing best available therapy with best available therapy combined with early VST administration.
Ritchie: Novartis: Honoraria; BMS: Research Funding; Takeda: Research Funding; CRISPR Therapeutics: Research Funding; Amgen Inc: Honoraria, Research Funding; CSL: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal